Are therapeutic nanoparticles dangerous?
Tiny nanoparticles are intended for transporting active substances to specific targets in the body or to heat tissue in cancer treatment. In short-term use these particles are stable and don’t damage cells but only little is known about possible long-term effects.
To treat serious disease such as cancer, there are ongoing efforts to pack treating substances into nanoparticles. In this form, the drug should then only be released at or inside the intended target, a tumor, for example, to achieve high local concentrations without causing strong side effects as is often the case with current treatment methods. Possible materials to transport the drugs are carbon and iron, the latter of which acts in a strictly physical manner. But how these particles behave in an organism? Are they themselves a possible health hazard? These exact questions have been tackled by scientists from the Heinrich-Heine University in Düsseldorf, Germany. They further investigated the mechanisms through which our bodies disposes of these tiny particles with a specific focus on carbon.
Carbon-nanoparticles in cells
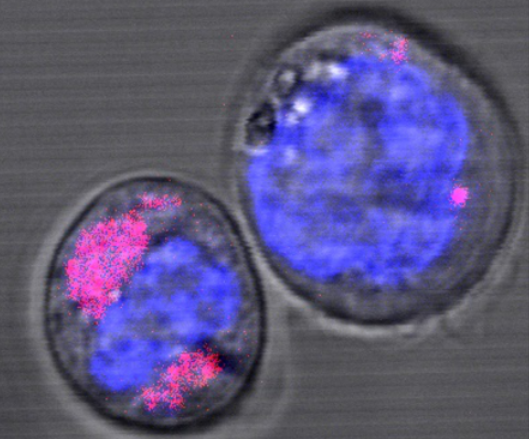
With any new treatment approach, the desirable effects are accompanied by potential risks. Due to their small size of less than five nanometers, such particles can cross the body’s main barriers. Mucous membranes are no obstacle for them. Even the blood-brain-barrier that seals off our brains from the rest of our body can be overcome by these particles, leading to the first problem: nanoparticles disperse throughout the entire body. To guide them to an intended target however, cancer cells need to express specific proteins on their surface, similar to a lock on a door. The nanoparticles then need to be equipped with the matching key to unfold its beneficial traits. Ideally, the particles reach the intended target where they release the active substance. The particle itself would remain inside the target cell. That this is possible has been proven by scientists from Düsseldorf, using graphene nanoparticles. These two-dimensional, hexagonal carbon structures were introduced to stem cells of the hematopoietic system, a cell species with high sensitivity. Any possible effects in these cells would be far greater than in other, more robust cells, the scientists say.
Particles are engulfed and disposed
The results show that the carbon nanoparticles indeed reached their intended target cells, but were then engulfed by lysosomes, a special organelle. Lysosomes are responsible for a cells waste management. They possess a biological membrane to form an acidic environment with various digestion enzymes. However, for the duration of the experiment, the scientists did not observe a degradation process, which is why it remains unclear what exactly happens to these carbon nanoparticles. If the entire cell dies however, the removal of the entire structure would be carried out by macrophages, but this process was not investigated in the present study.
There were some significant findings nonetheless. The tiny transport particles influenced gene activity only in negligible fashion. When comparing stem cells with and without nanoparticles, only one out of 20’800 analyzed sequences was significantly altered, while another 1171 resulted in minor changes.
“Because the nanoparticles were encapsulated by lysosomes, the particles were not able to cause damage to the cell, during the duration of our experiments”, Thomas Heinzel, Professor at Universität Düsseldorf, explains. “The cell’s viability without any major changes in gene expression is therefore ensured.” Possible long-term effects such as an increased likelihood of malignant cell degeneration could not be investigated with the experiment however.
A question of materials
Heinzel and his colleagues investigated the effects of carbon. For brain tumors, iron oxide nanoparticles are the most discussed however. They are administered via injection, then pass the blood-brain-barrier and concentrate in the cancerous tissue due to special coatings on the particle surface. Subsequently applying an alternating magnetic field will cause the particles to heat up quite strongly. This applied heat in turn is then supposed to destroy the tumor or make it more susceptible to the chemotherapeutic drugs. The iron oxide does not pose a problem because the employed doses are not toxic and the substance can be degraded by the body. Iron is also found, for example, in large quantities in red blood cells. Long-term effects are not thought to be of any concern because depending on the type, the brain tumor can be highly aggressive. If patients survive only a couple months longer, this already constitutes a success.
Source (German only): https://www.ingenieur.de/technik/fachbereiche/nanotechnologie/wie-gefaehrlich-sind-therapeutische-nanopartikel/
Original article and image source: "The Low Toxicity of Graphene Quantum Dots is Reflected by Marginal Gene Expression Changes of Primary Human Hematopoietic Stem Cells"