Iron oxide nanoparticles for oral drug delivery
Iron oxide nanoparticles are finding a rapidly increasing number of biomedical applications. However, a wide variety of safety concerns, especially those related to oral exposure, still need to be addressed in order to reach clinical practice. A recently published study investigated the effects of chronic oral exposure to iron oxide nanoparticles in growing chicken. The findings revealed that oral administration of iron oxide nanoparticles is a safe route for drug delivery at low nanoparticle doses.
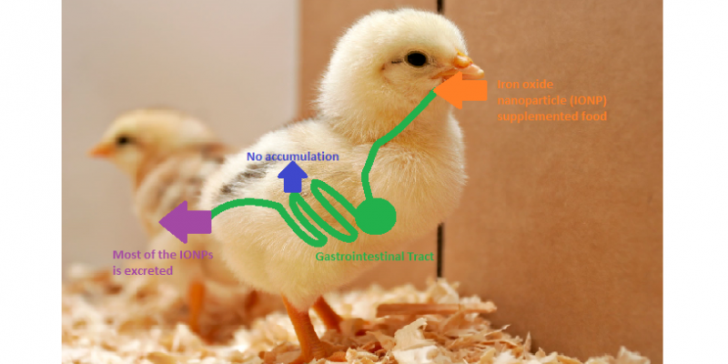
Iron oxide nanoparticles (IONP) provide a variety of possible medicinal applications, for example drug delivery. The modification of synthetically produced nanoparticles, in the case of this study magnetized particles, allows intentional drug release into the target tissue. The use of such engineered nanoparticles is expected to increase. However, results of previous studies with regard to toxicity and bioavailability of IONP are controversial, some indicating toxicity and bioavailability of IONPs. Thus, prior to any incorporation of IONP into clinical practice a safety assessment of IONP after a chronic oral exposure is mandatory. The aim of the study was therefore to clarify these results and investigate absorption pathways of IONP in the gastrointestinal tract.
The study applied broiler chicken as test animal, which were divided into three groups fed with three different diets. One group of chicken got iron supplemented food, one group got food supplemented with a low (<300 mg kg-1) dose of IONP, and third group worked as control group (no iron at all). After an adaptation time, a dietary period of 14 days followed. Then, blood samples of the chicken were taken and their weight was measured. Subsequently, tissue samples of duodenum, liver and spleen were taken. In addition, excremental samples were collected. The authors investigated iron accumulation in organ tissue and excrements and conducted statistical tests correlating the iron amounts found with the dietary iron dose. Further, it was analysed how IONP degrades in a gastrointestinal-similar environment and how the product of degradation (Fe(II)) was taken up into the blood circle. Therefore morphology of duodenum cells was analysed. Finally, a gene expression analysis was conducted with DNA from duodenum tissue.
No signs of toxicological effects, such as mortality or reduced weight gain, could be found among the chicken fed with IONP. Also, no significant amounts of IONP accumulated in liver and spleen tissue and the analysis of faeces showed that large amounts of IONP were excreted. Additionally, solubility of IONP in a simulated gastrointestinal tract was very low, showing only small amounts of ferric ions (FE (III)) released. This indicates low degradation of the particles. Also, iron accumulation was similar for non-iron and IONP fed animals suggesting no accumulation in duodenum, in contrast to chicken fed with iron supplements. This is not surprising, as these degrading iron supplements release ions (Fe (II)) that are known to be more soluble. Further, no effects could be found on the number of red blood cells and other haematological parameters. An additional analysis showed that duodenum epithelium is not affected, but that the IONP fraction that is degraded would be bioavailable.
Thus, this study provides by now evidence that oral administration of a low dose of iron oxide nanoparticles is safe.
Source: IOPScience (2015)
Study: Chamorro, S. et al. Safety assessment of chronic oral exposure to iron oxide nanoparticles, Nanotechnology (26), (2015).
Image source: Adapted from Fir0002 (Wikimedia Commons)