Fight tiny with tiny
Microorganisms are constantly evolving, as we know all too well today from SARS-CoV-2. Every mutation has the potential to bring new dangers to humans. This also gives rise to antibiotic resistance, a global health problem that concerned us even before the pandemic. Two independent research teams are now using the technology of the tiny to fight dangerous tiny organisms.
With the COVID-19 pandemic (still ongoing), infectious diseases have once again been increasingly on society's radar. In developed countries, other non-communicable diseases cause more suffering, but the current situation has reminded everyone of our tiny roommates: Viruses, bacteria and fungi. For some time now, antibiotic resistance in particular has been a pressing problem. In mankind's arms race against bacteria, some bacteria are currently ahead. Our former wonder weapons, antibiotics, are increasingly failing germs that use tricky maneuvers to protect themselves from the drugs' effects.
Staphylococci, for example, are mostly harmless germs that can occur on the skin and mucous membranes. Under certain conditions, however, the bacteria flood the body and trigger severe inflammation, even toxic shock or blood poisoning. This makes staphylococci the leading cause of death from infections with just one type of pathogen.
Particularly precarious is the increasing number of staph infections that no longer respond to treatment with antibiotics. MRSA, multi-resistant germs, are particularly feared in hospitals, where they cause poorly treatable wound infections as nosocomial pathogens or colonize catheters and equipment. In total, around 75,000 hospital infections occur in Switzerland every year, 12,000 of which are fatal. Worldwide, antibiotic-resistant pathogens cause an estimated 700,000 deaths - a global health challenge.
While the health burden of fungal infections is less well known, they kill about 1.5 million people worldwide each year, and the number of deaths is rising. For example, an emerging threat to hospitalized COVID-19 patients is the common fungus Aspergillus, which can cause deadly secondary infections.
Researchers are therefore exploring ways to combat the tiny pathogens with equally tiny technologies.
Bioglass and metal destroy tricky germs
Among bacteria, there are some particularly tricky pathogens that invade body cells, where they are invisible to the immune system. In this way, they outlast periods when the body's defenses are on alert. This phenomenon is also known for staphylococci. They can retract into cells of the skin, connective tissue, bone and immune system. The mechanism of this persistence is not yet fully understood.
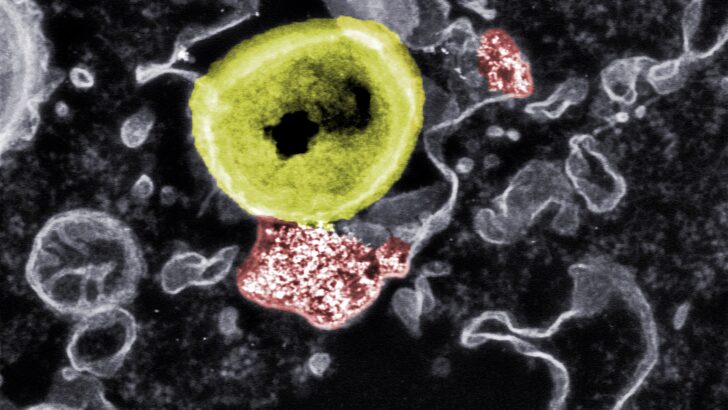
To track down the germs in their hiding place and render them harmless, a team of researchers from Empa and ETH Zurich has now developed nanoparticles that use a completely different mechanism of action than conventional antibiotics: Whereas antibiotics have difficulty penetrating body cells, these nanoparticles, due to their small size and composition, manage to be smuggled into the interior of the affected cell. Once there, they fight the bacteria.
For this purpose, the team led by Inge Herrmann and Tino Matter used the material cerium oxide, which in its nanoparticle form has antibacterial and anti-inflammatory effects. The researchers combined the nanoparticles with a bioactive ceramic material known as bioglass. Bioglass is interesting for medicine because it has versatile regenerative properties and is used, for example, for the reconstruction of bones and soft tissues.
Cerium - All-rounder among the chemical elements
The chemical element cerium, or cerium, was unjustly named after the dwarf planet Ceres. Because the silvery metal is currently making a big splash. As cerium oxide, it is used in automotive catalytic converters and in the manufacture of such diverse products as self-cleaning ovens, windshields and light-emitting diodes. Its antimicrobial and anti-inflammatory properties also make it interesting for medical applications.
Finally, nanoparticle hybrids of cerium oxide and bioglass were produced by flame synthesis. The particles have already been successfully used as wound adhesives, whereby several interesting properties can be exploited simultaneously: Thanks to the nanoparticles, bleeding can be stopped, inflammation dampened and wound healing accelerated. In addition, the novel particles show a significant effect against bacteria, while the treatment is well tolerated by human cells. Only recently, the new technology was successfully patented. The team has now published their results in the scientific journal "Nanoscale" in the "Emerging Investigator Collection 2021". Tino Matter is currently working on bringing the new technology to market. His startup anavo medical has already celebrated several successes - among others, it was among the three finalists of the Swiss Technology Awards.
The researchers were able to demonstrate the interactions between the hybrid nanoparticles, the body's cells and the germs using electron microscopy studies, among other things. When infected cells were treated with the nanoparticles, the bacteria inside the cells began to dissolve. If, on the other hand, the uptake of the hybrid particles was specifically blocked by the researchers, the antibacterial effect also stopped.
The exact mechanism of action of the cerium-containing particles is not yet fully understood. It has been proven that other metals also exhibit antimicrobial effects. Cerium, however, is less toxic to body cells than silver, for example. The researchers currently assume that the nanoparticles act on the cell membrane of the bacteria, producing reactive oxygen compounds that lead to the destruction of the germs. Since the membrane of human cells has a different structure, body cells are spared this process.
Against such a mechanism, the researchers believe, fewer resistances would presumably be able to develop. "In addition, the cerium oxide particles regenerate again over time, so that the oxidative effect of the nanoparticles on the bacteria sets in again," says Empa researcher Tino Matter." In this way, the cerium particles could have a lasting effect.
Next, the researchers want to analyze the interactions of the particles in the infection process in more detail in order to further optimize the structure and composition of the nanoparticles. The goal is to develop a simple, robust antibacterial agent, effective inside infected cells.
Nanoscale-thin black phosphorus disrupts microorganisms
A new antimicrobial coating from a team led by RMIT University is based on an ultrathin 2D material that has been of most interest in next-generation electronics, black phosphorus, or black phosphorous (BP) in English. The material is one of the thinnest antimicrobial coatings developed to date. It is effective against a broad spectrum of drug-resistant bacteria and fungal cells while leaving human cells intact.
Co-lead researcher Associate Professor Sumeet Walia of RMIT's School of Engineering has previously led groundbreaking studies using BP for artificial intelligence technology and brain-mimicking electronics.
"BP decays in the presence of oxygen, which is usually a big problem for electronics and something we had to overcome with painstaking precision engineering to develop our technologies," Walia said.
"But it turns out that materials that decompose easily with oxygen can be ideal for killing microbes - it's exactly what scientists working on antimicrobial technologies were looking for. "So our problem was their solution."
Studies on BP have suggested that it has some antibacterial and antifungal properties, but the material has never been methodically studied for potential clinical use.
The new study, published in the American Chemical Society journal Applied Materials & Interfaces, shows that black phosphorus effectively kills microbes when applied in nano-thin layers to surfaces such as titanium and cotton used to make implants and wound dressings.
Co-lead researcher Dr. Aaron Elbourne said finding a material that can prevent both bacterial and fungal infections is a significant advance.
"These pathogens are responsible for massive health burdens, and as drug resistance continues to increase, it's becoming increasingly difficult for us to treat these infections," said Elbourne, a postdoctoral fellow in RMIT's School of Science.
"Our nanothin coating is a double bug killer that works by ripping apart bacterial and fungal cells, something that microbes have a hard time adapting to. It would take millions of years for new defenses to naturally evolve against such a deadly physical attack.
"While we need more research to apply this technology in clinical settings, this is an exciting new direction in the search for more effective ways to address this serious health challenge."
When BP decays, it oxidizes the surface of bacteria and fungal cells. This process, known as cellular oxidation, eventually causes them to be torn apart.
In the new study, first author and doctoral student Zo Shaw tested the effectiveness of nanothin coatings made from BP against five common strains of bacteria, including E. coli and drug-resistant MRSA, and five types of fungi, including Candida auris.
In just two hours, up to 99% of bacterial and fungal cells were destroyed.
Importantly, the BP also began to self-degrade during this time and was completely degraded within 24 hours - an important feature that shows the material is not accumulating in the body.
The laboratory study identified the optimal levels of BP that would have a lethal antimicrobial effect while leaving human cells healthy and unharmed.
The researchers have now begun experimenting with different formulations to test efficacy on a range of medically relevant surfaces.
The team is keen to work with potential industry partners to further develop the technology, for which a provisional patent application has been filed.
Editor's note: This text was merged from two separate articles (see sources).
Editor: Alex von Wyl
Original publications:
ACS Applied Materials Interfaces: Shaw et al. (2021) – Broad-Spectrum Solvent-Free Layered Black Phosphorous as a Rapid Action Antimicrobial
Nanoscale: Matter et al. (2021) – Inorganic nanohybrids combat antibiotic-resistant bacteria hiding within human macrophages
Sources:
Phys.org – Superbug killer: New nanotech destroys bacteria and fungal cells
EMPA – Mit Nanopartikeln gegen gefährliche Bakterien
Image source: EMPA